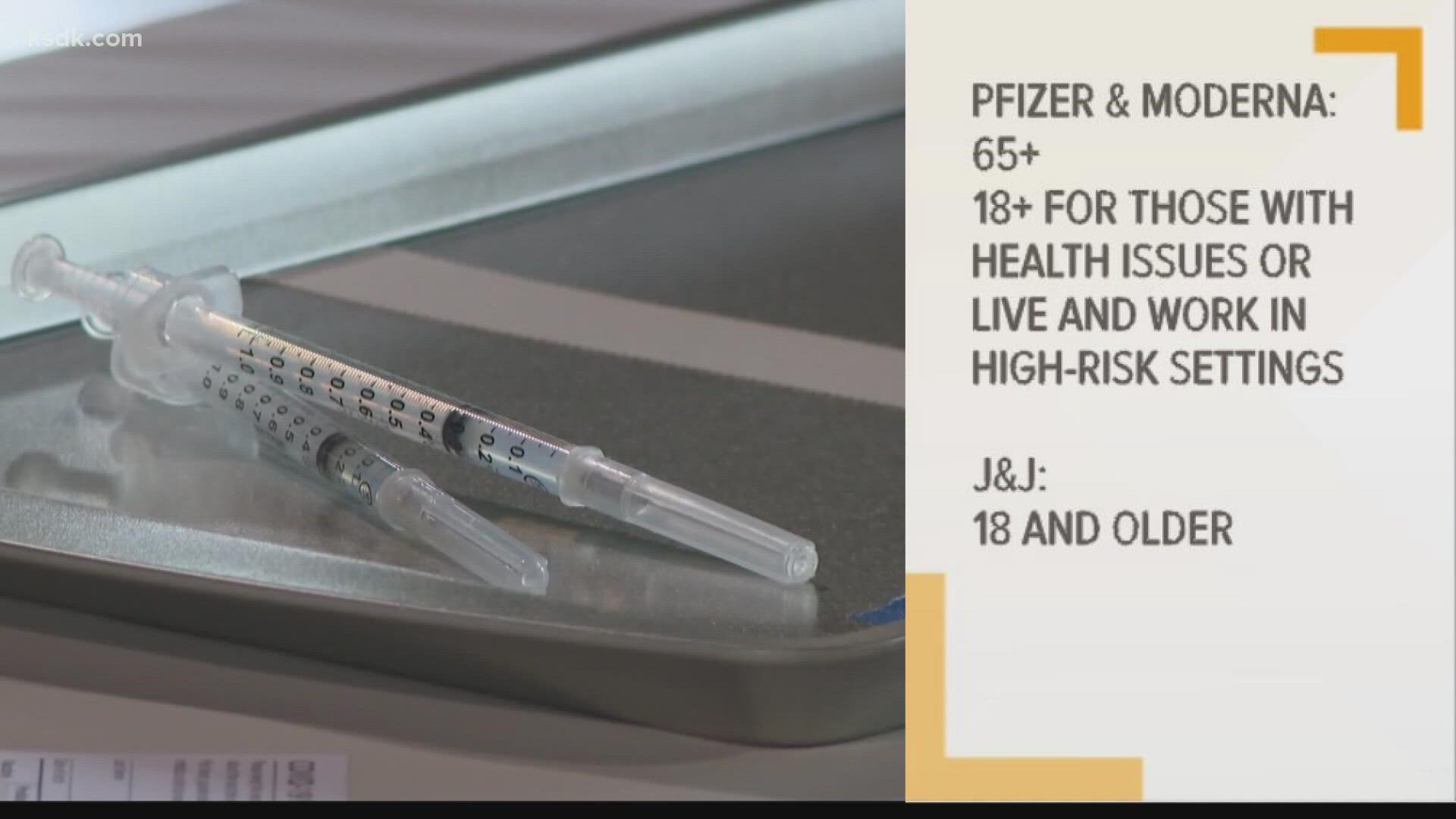
Moderna Booster Shot
1 day agoThe European Unions drug regulator expects to announce the results of its review of Modernas COVID-19 booster vaccine next week and also to start a rolling review of Mercks antiviral oral pill. The longest duration the Pfizer and Moderna vaccines have been studied for is six months so experts know very little about how the vaccine behaves beyond that.
Moderna Says Higher Infection Risk After One Year Supports Covid Boosters Barron S
The Moderna booster shots are authorized under the same terms including having to wait until six months after the second dose but JJ vaccine recipients can get any booster two months after.

Moderna booster shot. 5 hours agoThe FDA authorized a Moderna vaccine booster for Moderna recipients age 65 years of age and older and adults who are at high risk because of severe illness or exposure in their work setting. In the end the FDA. In September 2021 the Food and Drug Administration.
M any Americans who have been vaccinated against Covid-19 will soon be able to choose which vaccine they would like as. 2 days agoThe use of a single booster dose of the Moderna COVID-19 Vaccine that may be administered at least 6 months after completion of the primary series to. COVID-19 vaccine maker Moderna will make a third booster shot for its two-dose vaccine available to Americans by the fall CEO Stéphane Bancel said this week.
WASHINGTON AP US. Vaccine developers at Moderna are looking forward towards our vision of a single dose annual booster that provides protection against COVID-19 flu and respiratory syncytial virus for adults. 2 days agoLike with Pfizers vaccine the FDA is authorizing a third Moderna shot for many adults vaccinated at least six months ago.
The risk of severe illness from COVID-19 increases with age and can also increase. While experts have made their best predictions based on what they know the decisions surrounding booster shots rely on data that has not yet been acquired. 1 day agoThe Centers for Disease Control and Prevention late Thursday cleared booster shots of Modernas and Johnson Johnsons Covid-19 vaccines giving people the freedom to mix and match any of the.
JJ said a. If you are fully vaccinated with the Pfizer vaccine and received the second dose at least six months ago you might be eligible for a booster shot now. 3 hours agoFILE This Thursday Feb.
The new CDC guidelines apply just to those. 8 hours agoPfizer whose vaccine is similar to Modernas said internal data showed that a booster dose of its vaccine restored protection against severe disease from Covid back to more than 95. Regulators on Wednesday signed off on extending COVID-19 boosters to Americans who got the.
Safety data of the half-dose Moderna booster is limited but the. 25 2021 file photo shows vials for the Moderna and Pfizer COVID-19 vaccines at a temporary clinic in Exeter NH. 2 days agoFDA approves Moderna JJ booster shots also says its OK to mix vaccines.
1 day agoBut Moderna only sought permission for a half-dose as a booster shot submitting data in support of that. People aged 65 years and older and adults 5064 years with underlying medical conditions should get a booster shot of Pfizer-BioNTech vaccine. FDA authorizes booster shots of Moderna Johnson Johnson Covid-19 vaccines.
By now you know that if youre getting the Moderna or Pfizer COVID vaccine youre scheduled for two doses while with Johnson. Decided to authorize the half-dose Moderna booster for recipients. Modernas vaccine is more than 90.
Moderna CEO Says a Booster Shot Will Be Available by This Date. The risk of severe illness from COVID-19 increases with age and can also increase. 2 days agoModerna said it chose the lower-dose booster because it triggered fewer uncomfortable shot reactions such as fever and achiness but also leaves more vaccine available for the global supply.
People aged 65 years and older and adults 5064 years with underlying medical conditions should get a booster shot of Pfizer-BioNTech vaccine. 23 hours agoThe Moderna shots tied to that risk were full doses not the half-dose booster cleared by the FDA and recommended by the CDC panel.
Moderna Plans To Have Covid Vaccine Booster Shot Ready By Fall Cbs News

Fda Oks Mixing Covid Vaccines Backs Moderna J J Boosters Woodtv Com
Fda Authorizes Moderna Johnson Johnson Booster Shots For Many Americans Cnet
Covid Booster Shots Everything You Need To Know The Brink Boston University
Covid Booster Shot Moderna Says Vaccine Generates Promising Immune Response Against Variants

European Agency Clears Moderna Vaccine For Children 12 17 Wvns

Covid 19 Booster Shots To Be Availed For More People In The Us Check Who Can Get The Vaccine Booster Shots The Financial Express
Moderna Covid Vaccine Booster Shot The Latest On Approval Process Cnet
Moderna To Supply 20 Mln Doses Of Covid 19 Vaccine To Peru Reuters
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/gray/NZPGPXPQUJDSVC7FJIONPNSKWU.jpg)
Can People Who Received Moderna Or Johnson Johnson Get A Pfizer Booster Shot Health Officials Explain

Boosters For Moderna And J J Recipients Not Up For Debate At C D C Panel The New York Times
Covid Vaccine Ema Backs Pfizer Or Moderna Booster Shots For People With Weak Immunity Euronews

Moderna Is Developing Vaccine Booster Shot To Fight South Africa Covid Strain Youtube
Moderna Has Asked The Fda To Authorize A Booster Of Its Covid 19 Vaccine Wxxi News